Over the years, the flexiVent has become the industry standard in terms of preclinical lung function measurements and has proven to be an invaluable tool in early drug development, as it goes beyond traditional resistance and compliance measurements. The flexiVent has been used successfully and efficiently during the development of novel drug models, in gaining novel insights into the mechanism of action of drugs, in pre-clinical drug testing, and bench-marking marketed drugs in murine models.

SCIREQ has put together a case study highlighting Dr. Phillips’ group at Amgen on the use of in vivo models for the drug development process.
He reviewed the history of reslizumab, a humanized monoclonal antibody with IL-5 neutralizing effects that reduced circulating eosinophils, but failed to show improvements in lung function during a costly clinical trial.
In a post-mortem project analysis, Dr. Phillips ran a separate study where he measured pulmonary resistance in mice using the flexiVent and concluded that the results from the reslizumab trial were predictable preclinically.
Read the full case study here
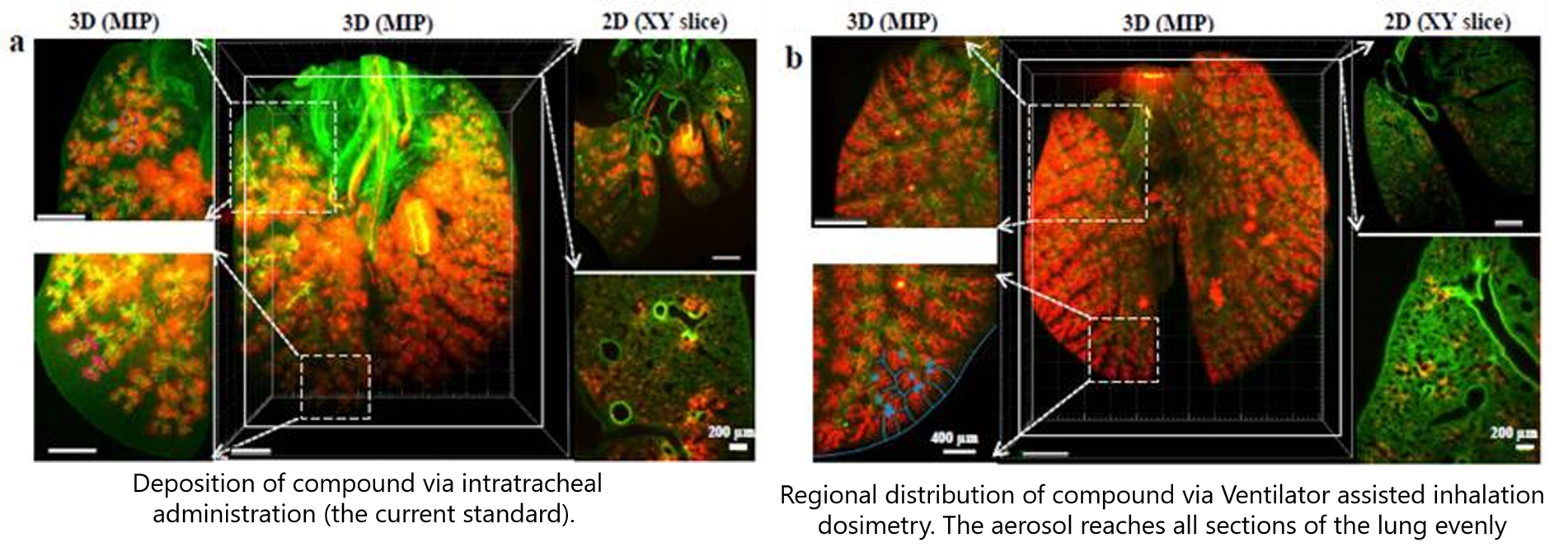
Ventilator-assisted aerosol delivery is a hybrid solution that bridges the gap between instillation and inhalation. One of our collaborators from Germany, Dr. Schmid, has published beautiful light-sheet microscopy images that convey how this approach can deliver aerosols efficiently and with homogenous deposition profiles using the flexiVent.
Dr. Schmid also recently posted a video of fluorescent nanoparticles beautifully distributed throughout a mouse’s lung using these methods
Read how the flexiVent compares to other drug delivery methods


SCIREQ Scientific Respiratory Equipment Inc.
Head Office : 6600 St-Urbain Suite 300 Montreal, QC H2S 3G8 Canada
Telephone: Toll free 1 (877) 572-4737 or 1 (514) 286-1429
info@scireq.com